Science: The Power of the Atom
 |
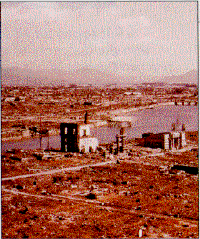
| On August 6, 1945, a single atomic bomb was dropped on Hiroshima, Japan, devastating most of the city (below). Photo of the mushroom cloud (above) was taken several minutes after bomb was detonated. |
 |
Einstein’s theories shattered some time-honored assumptions about the nature of matter. He showed that mass and energy are interrelated. Like ice and steam, they are different forms of the same thing and in certain circumstances are interchangeable.
Scientists had known that matter could produce energy as a result of a chemical reaction. For example, if you were to set some paper on fire, it would produce heat, light and some sound. But this chemical process would release only a small percentage of the paper’s total energy potential. If the reaction were to take place at the subatomic level, the amount of energy released would be enormous.
To accomplish this, the mass of the atoms that make up the elements of the paper would need to become “unfrozen” and turned into energy. The energy could then be calculated by the famous equation E = mc2 (where m is mass and c2 is the speed of light squared).
If all the energy compacted in a small booklet could be released (it can’t by any means known to science), it would supply enough energy to run a city about the size of San Diego, California, for 99 days. As you can see, the energy potential of matter is enormous!
There was a dramatic demonstration of the power locked in the heart of the atom on Aug. 6, 1945. About 22 pounds (10 kilograms) of uranium were used in the atomic bomb that exploded over Hiroshima, Japan. Of that, it has been estimated that only a fraction of the mass of the uranium (about the size of a pea) was converted into energy. Humanity had learned how to unleash the awesome power of the atom.
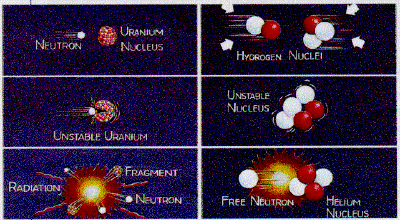 |
Nuclear fission and nuclear fusion are two kinds of nuclear reactions that release tremendous amounts of energy. Nuclear fission involves the splitting of a heavy element, such as uranium, to release energy (far left). Nuclear fusion occurs when two lightweight nuclei combine and form a nucleus of a heavier element (right column). Repeated many times, fusion creates the energy of the sun and the hydrogen bomb. | |
| Fission | Fusion | |
It is the awesome power of nuclear energy that fuels the stars. The massive fusion reactions that take place deep within our sun convert about 4 million tons (3.7 million metric tons) of the sun’s mass into energy every second. The sun is just one medium-sized star in our galaxy of some 100 billion other stars. Our galaxy is just one of an estimated 100 billion other galaxies, each having perhaps 100 billion stars. And, on average, each is converting matter into energy at the rate of millions of tons every second. Remember, it took just a pea-sized fraction of uranium in an atomic bomb to destroy Hiroshima.
Just how much power is out there?

